Breaking Azeotropes and Absorption Tricks
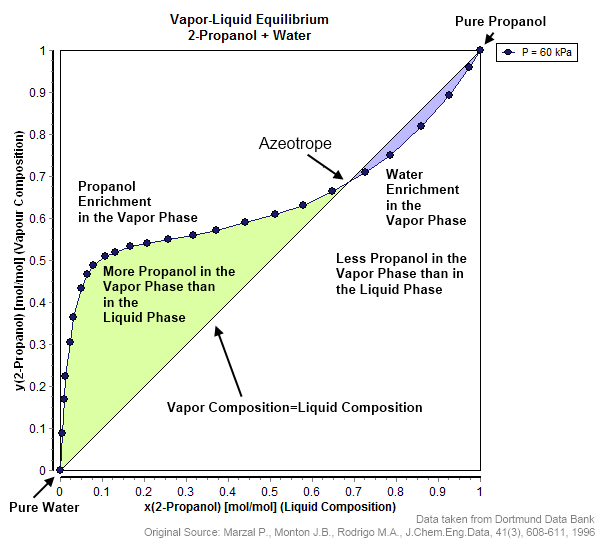
- In many azeotropic systems, pressure swing distillation is ineffective.
- For example, in the ethanol-water system above, the relative volatility for the high concentrations of ethanol is close to α≈1 !
- What other methods are available for breaking azeotropes?
-
To break this azeotrope, we must either:
- Modify the VLE behaviour of the mixture using another component.
- Use an alternative separation technique.
- Alternative separation techniques may be used to just to completely separate the azeotropic mixture, or just to help distillation get past the azeotrope.
- The final technique we'll mention is the use of an entrainer.
- This is an additional component added to the column to alter the VLE behaviour of the mixture.
- In Extractive distillation the entrainer is a non-volatile component that exits in the bottom product and binds strongly to only one of the components.
- This technique can be thought of as a combination of liquid-liquid extraction and distillation occurring in one column at the same time.
- In azeotropic distillation, an entrainer is added which is volatile and exits the column in the top-product.
- This method is called azeotropic distillation as a new ternary azeotrope is often formed, but this azeotrope will be at lower concentrations than the binary azeotrope.
- The distillation can then be continued up to high purities, but a recovery strategy must be devised to recover the entrainer.
- This technique is quite popular for the dehydration of ethanol…


- In multi-stage absorbtion, we have an operating line of the following form. L′xA,i1−xA,i+V′yA,j+11−yA,j+1=L′xA,j1−xA,j+V′yA,i+11−yA,i+1
- We also have some equilibrium data, which at typical absorber concentrations can described by Henry's law y=H x
- The solution of these equations is just the same as for multi-stage distillation. We draw the operating line and the equilibrium line and step between the two.
L′xA,i1−xA,i+V′yA,j+11−yA,j+1=L′xA,j1−xA,j+V′yA,i+11−yA,i+1
- One of the biggest difficulties of absorber design is that you have to alter the operating line a lot.
- In distillation, the operating line is straight due to the assumption of constant molar overflow.
- But in absorption, we must plot several points to capture its curvature (see right).
- Can we make the operating line straight using a change of variables?
- Defining two new variables: X=x1−xY=y1−y
- We can rewrite the operating line in terms of these variables. L′Xi+V′Yj+1=L′Xj+V′Yi+1
- Rearranging then gives us a convenient linear form: YA,j+1=L′V′(XA,j−XA,i)+YA,i
- We can easily convert back from these new variables too: y=Y1+Yx=X1+X
- This form is much more convenient to plot, as it is a straight line on a XY plot.
- It is also very easy to convert from x→X or y→Y.
- The only difficulty is that the equilibrium line is no longer straight. y=H xY=H x1+H x
- However, if XY graphs for the equilibrium data have been prepared once, it is much more convenient to perform the design in these variables.
Breaking Azeotropes and Absorption Tricks